Circadian Rhythm Influences Blood Cholesterol
Misha Naiman
May 15, 2017
Environmental disruption of the circadian rhythm can lead to altered metabolic function, while modern lifestyles lead people into asynchrony between external time and internal phasic time. The disruption of circadian rhythm and altered metabolism can lead to an increase in obesity, diabetes and other life threatening metabolic disease. This review by Reinke and Asher provides an overview on how the gastrointestinal tract and the liver are under circadian modulation, focusing on the circadian role in metabolism and degradation of lipids, bile acids and cholesterol.
The body’s circadian clock is regulated by a master pacemaker in the superchiasmatic nucleus (SCN) in the hypothalamus, which receives it’s time cues from light exposure through the retinahypothalamic tract. Secondary pacemakers in peripheral organs, such as the liver, get their cues through energy sensing systems like AMPK and metabolic feedback loops, making these clocks extremely responsive to metabolic cues and feeding behaviors. Reinke and Asher state that the peripheral clock within the liver can be efficiently entrained by an individual’s fast/feed cycle to the point of being completely separate from the master rhythms generated by the SCN (Fig 1.).
Fig. 1. The master clock in the SCN synchronizes with geophysical time using light cues and temperature cycles; it communicates this information to peripheral oscillators, including the liver clock, which regulate the cyclic expression of rate-limiting metabolic enzymes. The bile acid metabolism, cholesterol biosynthesis and lipid metabolism cycles are of key interest to us in the blood tester group (Rienke and Asher, 2016).
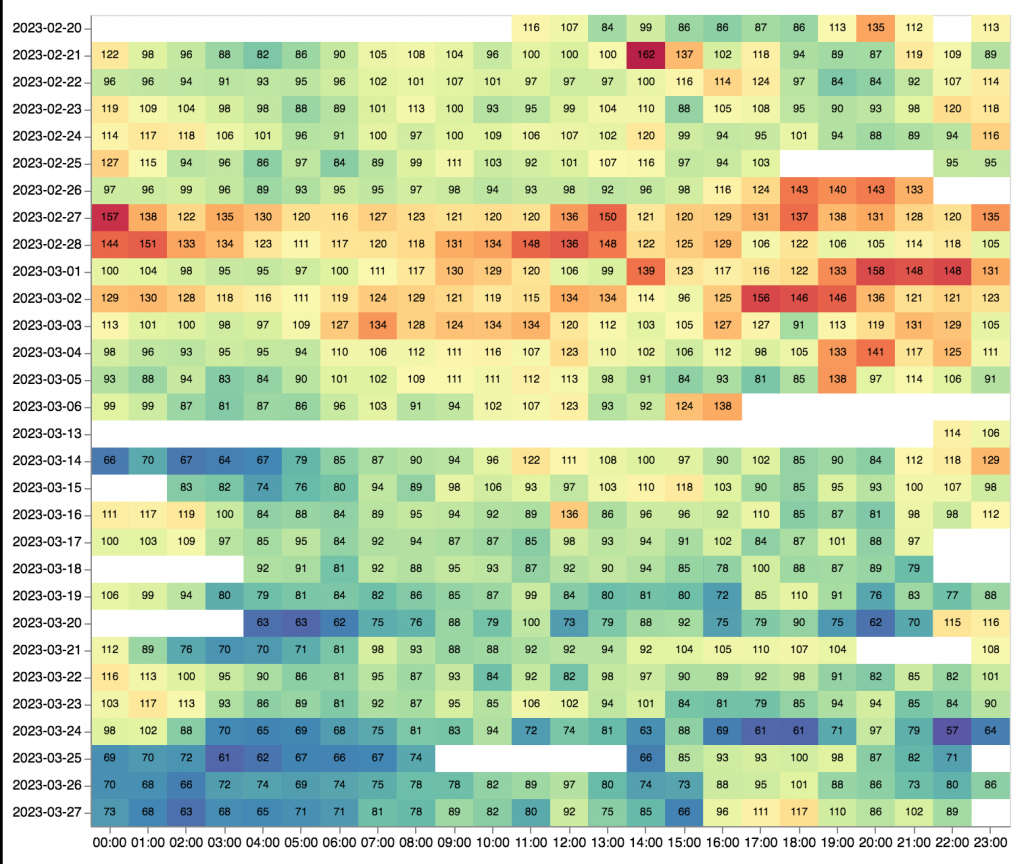
The liver clock is largely responsible for buffering the circadian fluctuation of blood glucose and cholesterol in response to an individual’s eating habits. The conversion of cholesterol to mevalonate acid by HMGCoA reductase (Hmgcr) has been known to be circadian clock regulated since groundbreaking work by Shapiro and Rodwell in 1969. Panda et al. expand on Shapiro and Rodwell’s work by demonstrating that many key enzymes in the cholesterol pathway exhibit coordinated expression; Enzymes responsible for cholesterol biosynthesis like Hmgcr peak during the night, when dietary cholesterol is low, and enzymes of cholesterol degradation are expressed evenly at different times of the day (fig. 2B and 2C).
Fig. 2. A) An overview of the cholesterol biosynthesis in mammals. The liver is responsible for both the synthesis and degradation of cholesterol, which it breaks down into different hormones and bile acids. B) Expression pattern of enzymes (including Hmgr and Npc1) involved in cholesterol synthesis. **X-axis represent hours after first subjective dawn.** C) The degradation products of cholesterol, bile acids and hormones, are produced evenly throughout the subject’s day (Panda et al., 2016).
The circadian rhythm regulates many aspects of hepatic lipid metabolism; conversely dietary lipid intake and feeding behaviors can act as a potential regulators of circadian rhythm (Reinke and Asher, 2016). A recent lipidomic analysis of WT mice and CLOCK ko mice (CLOCK being an essential transcription factor in the peripheral oscillatory pathway that generates circadian rhythm) demonstrated that CLOCK ko mice had significantly different blood lipid composition and circadian phase shift (Adamovich et al. 2014).
The liver is the primary organ responsible for converting cholesterol into bile acids which aides in the digestion and absorption of fats and oils. Cholesterol is primarily degraded into bile acids by the rate-limiting enzyme CYP7A1. The transcription of this enzyme relies on a molecular cascade involving the bile acid receptor FXR and the promoter specific activator SHP (Lu et al. 2000). Recently, Le Martelot et al. discovered a strong link between NR1D1, a transcription factor involved in the circadian circuitry, and transcription of CYP7A1. This demonstrates that the circadian clock acts as an additional regulatory force in cholesterol degradation, working alongside the molecular cascade (Le Martelot et al. 2009)
Adamovich, Yaarit, Rona Aviram, and Gad Asher. “The emerging roles of lipids in circadian control.” *Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids* 1851.8 (2015): 1017-025. Web. Adamovich et al. review recent publications regarding lipid involvement in circadian clock research, as mounting evidence suggest that lipids are embedded within circadian clock circuitry in the SCN and the liver. Rapid technological advances has improved lipidomics methodologies which has shed new light on the interplay between circadian rhythm and lipid metabolism.
Lu, Timothy T., Makoto Makishima, Joyce J. Repa, Kristina Schoonjans, Thomas A. Kerr, Johan Auwerx, and David J. Mangelsdorf. “Molecular Basis for Feedback Regulation of Bile Acid Synthesis by Nuclear Receptors.” *Molecular Cell* 6.3 (2000): 507-15. Web. This paper demonstrates that the catabolism of cholesterol into bile acids is dependent on an autoregulatory cascade involving a triad of nuclear receptors:FXR, LRH-1 and SHP. Bile acid biosynthesis is dependent on the repression of rate-limiting enzyme, CYP7, which is modulated primarily by FXR, LRH-1 and SHP and secondarily by peripheral clock circuitry.
Martelot, Gwendal Le, Thierry Claudel, David Gatfield, Olivier Schaad, Benoît Kornmann, Giuseppe Lo Sasso, Antonio Moschetta, and Ueli Schibler. “REV-ERBα Participates in Circadian SREBP Signaling and Bile Acid Homeostasis.” *PLoS Biology* 7.9 (2009): n. pag. Web.
REV-ERBa, an important component of circadian clockwork circuitry, modulates the rhythmic metabolism of cholesterol and bile acid biosynthesis in the liver. Previously it was believed that dietary cholesterol was the primary trigger for bile acid synthesis. However transcriptome profiling experiments with liver RNA from WT mice, REV-ERBa ko mice and REV-ERBa overexpressing mice found that REV-ERBa participates in the modulation of cholesterol metabolism. Most notably, REV-ERBa triggers the expression of CYP7A1, the rate-limiting enzyme in cholesterol catabolism.
Panda, Satchidananda, Marina P. Antoch, Brooke H. Miller, Andrew I. Su, Andrew B. Schook, Marty Straume, Peter G. Schultz, Steve A. Kay, Joseph S. Takahashi, and John B. Hogenesch. “Coordinated Transcription of Key Pathways in the Mouse by the Circadian Clock.” *Cell* 109.3 (2002): 307-20. Web. Conducted a large scale gene expression profiling experiment to identify key cycling transcripts involved in circadian rhythm. They found 650 cycling transcripts, of which a majority were expressed in SCN or liver tissue. Many major processes in the SCN and liver are regulated by circadian circuitry, highlighting the foundational role that the circadian clock plays in organismal physiology.
Reinke, Hans, and Gad Asher. “Circadian Clock Control of Liver Metabolic Functions.” *Gastroenterology* 150.3 (2016): 574-80. Web. Environmental disruption of the circadian rhythm can lead to altered metabolic function, while modern lifestyles cause people into asynchrony between external time and internal phasic time. The disruption of circadian rhythm and altered metabolism can lead to an increase in obesity, diabetes and other life threatening metabolic disease. This review provides an overview on how the gastrointestinal tract and the liver are under circadian modulation, focusing on its role in lipid, bile acid and cholesterol metabolism and degradation.
( Not cited in this blurb, but these articles are also great!)
Storch, Kai-Florian, Ovidiu Lipan, Igor Leykin, N. Viswanathan, Fred C. Davis, Wing H. Wong, and Charles J. Weitz. “Extensive and divergent circadian gene expression in liver and heart.” *Nature* 417.6884 (2002): 78-83. Web.
Wang, F., X. Zhang, J. Wang, M. Chen, N. Fan, Q. Ma, R. Liu, R. Wang, X. Li, M. Liu, and G. Ning. “LGR4 acts as a link between the peripheral circadian clock and lipid metabolism in liver.” *Journal of Molecular Endocrinology* 52.2 (2013): 133-43. Web.